Ivermectin, Mebendazole and Fenbendazole as Cancer Treatments: Research, Protocols, and Controversies (2025)
Exploring the Science, Success Stories, and Cancer Protocols Behind Ivermectin and Fenbendazole in 2025
Contents:
Introduction
Ivermectin: Key Resources
Ivermectin Access and Controversies
Fenbendazole: Key Resources
Experimental Cancer Protocols
Mebendazole: Key Resources
New & Improved Joe Tippens Protocol
Research Gaps and Future Directions
Conclusion
See updated guide:
Introduction
Cancer remains a leading cause of morbidity and mortality worldwide, with an increasing incidence of aggressive and treatment-resistant tumors such as triple-negative breast cancer (TNBC), pancreatic adenocarcinoma, and glioblastoma. Despite significant advances in targeted therapies and immunotherapies, many patients continue to face limited effective options, highlighting an urgent need for novel, affordable, and accessible treatment strategies.
The high cost of oncology drugs-exceeding $150 billion globally in 2022-and the slow pace of new drug approvals further complicate timely patient access to effective therapies. In this context, drug repurposing-the strategy of identifying new therapeutic uses for existing drugs-has emerged as a promising approach to accelerate cancer treatment development while reducing costs and safety risks.
Among repurposed candidates, antiparasitic drugs such as fenbendazole, mebendazole, and ivermectin have attracted considerable attention due to their demonstrated anticancer activities across multiple preclinical models and emerging clinical case reports. These agents, originally developed to treat helminth infections, exert multifaceted effects on cancer cells, including disruption of microtubule dynamics, interference with metabolic pathways, and modulation of oncogenic signaling.
Fenbendazole, a benzimidazole derivative widely used in veterinary medicine, has shown potent anticancer effects by destabilizing microtubules, inducing G2/M cell cycle arrest, and impairing glucose metabolism through inhibition of glucose transporters (GLUT1/4) and hexokinase activity. These actions lead to reduced glycolysis and lactate production, effectively starving cancer cells and overcoming drug resistance, particularly in 5-fluorouracil-resistant colorectal cancer models (Bai et al., 2009; Oral Fenbendazole for Cancer Therapy, 2024; Anti-cancer effects of fenbendazole on 5-fluorouracil-resistant cells, 2022). However, fenbendazole’s poor water solubility and limited oral bioavailability present challenges for achieving therapeutic systemic levels, necessitating formulation improvements and pharmacokinetic optimization.
Mebendazole, a structurally related benzimidazole with better bioavailability and a longer history of human use, similarly disrupts microtubule polymerization and induces apoptosis. It has demonstrated anticancer activity in diverse malignancies, including ovarian cancer, chronic myeloid leukemia, and glioblastoma, with evidence of synergistic effects when combined with tyrosine kinase inhibitors and other chemotherapeutics (Potential and mechanism of mebendazole, 2020; Anticancer potential of mebendazole against chronic myeloid leukemia, 2022; Repurposing Drugs in Oncology, 2014). Mebendazole’s ability to cross the blood-brain barrier further supports its investigation in brain tumors.
Ivermectin, a macrocyclic lactone antiparasitic, exhibits broad-spectrum anticancer effects through mechanisms distinct from benzimidazoles. It inhibits key oncogenic pathways such as STAT3, Wnt/β-catenin, and AKT/mTOR, induces oxidative stress, promotes apoptosis and autophagy, and targets cancer stem cells. Preclinical studies have demonstrated its efficacy across more than 20 cancer types, including breast, colon, lung, and hematologic malignancies, with promising activity against drug-resistant and metastatic tumors (OneDayMD, 2025; Ivermectin, a potential anticancer drug, 2021). Its favorable safety profile at standard doses supports combination regimens with fenbendazole and mebendazole, which may enhance therapeutic outcomes through complementary mechanisms.
Despite encouraging preclinical and anecdotal clinical evidence, these antiparasitic agents remain largely experimental in oncology, with limited randomized controlled trials* and regulatory approval for cancer indications. Variability in dosing protocols, access issues, and concerns about off-label use underscore the need for rigorous clinical evaluation. Nonetheless, their low cost, oral administration, and multi-targeted anticancer properties position fenbendazole, mebendazole, and ivermectin as attractive candidates for adjunctive cancer therapy, especially in resource-limited settings.
This guide compiles key resources—including research articles, protocols, and expert insights—exploring their possible roles in cancer therapy.
Ivermectin: Key Resources
Informative Article and Videos
June 10, 2024 - "15 minutes with Dr.Makis" - Episode 018: High Dose IVERMECTIN and CANCER
2025 Studies
Yuan Yuan et al (Cedars-Sinai Medical Center) - A phase I/II study evaluating the safety and efficacy of ivermectin in combination with balstilimab in patients with metastatic triple negative breast cancer (ASCO 2025)
Yuwen et al - A Review of Ivermectin Use in Cancer Patients: Is it Time to Repurpose the Ivermectin in Cancer Treatment?
Morinaga et al - Ivermectin Combined With Recombinant Methioninase (rMETase) Synergistically Eradicates MiaPaCa-2 Pancreatic Cancer Cells.
2024 Studies
Baghli et al - Targeting the Mitochondrial-Stem Cell Connection in Cancer Treatment: A Hybrid Orthomolecular Protocol. First-in-the-World Ivermectin, Mebendazole and Fenbendazole Protocol in Cancer has been peer-reviewed and published on Sep.19, 2024. Co-authors include Dr Paul Marik and Dr William Makis.
Fan et al - Ivermectin Inhibits Bladder Cancer Cell Growth and Induces Oxidative Stress and DNA Damage
Man-Yuan Li et al - Ivermectin induces non-protective autophagy by downregulating PAK1 and apoptosis in lung adenocarcinoma cells
Kaur et al - Ivermectin: A Multifaceted drug with a potential beyond anti-parasitic therapy
Xing Hu et al - Ivermectin as a potential therapeutic strategy for glioma
Yang Song et al - Gene signatures to therapeutics: Assessing the potential of ivermectin against t(4;14) multiple myeloma
Goldfarb et al - Lipid-Restricted Culture Media Reveal Unexpected Cancer Cell Sensitivities
Newell et al - Therapeutic targeting of nuclear export and import receptors in cancer and their potential in combination chemotherapy
One Day MD - IVERMECTIN and CANCER, it has at least 15 anti-cancer mechanisms of action. Can Ivermectin Treat COVID-19 mRNA Vaccine Induced Turbo Cancers? - 9 Ivermectin papers reviewed.
Types of Cancer Studied with Ivermectin
In Vitro and In Vivo Studies
Ivermectin has demonstrated anti-cancer activity in cell lines and animal models of:
Bladder Cancer - (2024 Fan et al) - Ivermectin Inhibits Bladder Cancer Cell Growth and Induces Oxidative Stress and DNA Damage.
Breast Cancer - (2018 Dominguez-Gomez et al) - Ivermectin as an inhibitor of cancer stem-like cells.
Brain Cancer - (2016 Liu et al) - Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress.
Bile Duct Cancer - (2019 Intyuod et al) - Anti-parasitic drug ivermectin exhibits potent anticancer activity against gemcitabine-resistant cholangiocarcinoma in vitro
Blood Cancer - (2020, de Castro et al) - Continuous high-dose ivermectin appears to be safe in patients with acute myelogenous leukemia and could inform clinical repurposing for COVID-19 infection.
Bone Cancer - (2022 Hu et al) - Repurposing Ivermectin to augment chemotherapy’s efficacy in osteosarcoma
Colon Cancer:
2022, Alghamdi et al - Efficacy of ivermectin against colon cancer induced by dimethylhydrazine in male wistar rats.
2025 Asano et al - Selective Synergy of Ivermectin Combined With Recombinant Methioninase Against Colon-Cancer Cells in Contrast to Normal Fibroblasts
Cervical Cancer - (2022, Qabbus et al) - Ivermectin-induced cell death of cervical cancer cells in vitro a consequence of precipitate formation in culture media
CML (Chronic Myeloid Leukemia) - (2018 Wang et al) - Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress.
Lung Cancer - (2024 Man-Yuan Li et al) - Ivermectin induces nonprotective autophagy by downregulating PAK1 and apoptosis in lung adenocarcinoma cells
Glioma - (2024 Xing Hu et al) - Ivermectin as a potential therapeutic strategy for glioma
Multiple Myeloma - (2024 Yang Song et al) - Gene signatures to therapeutics: Assessing the potential of ivermectin against t(4;14) multiple myeloma
Ovarian Cancer - (2023 Jawad et al) - Ivermectin augments the anti-cancer activity of pitavastatin in ovarian cancer cells
Prostate Cancer - (2022 Lu et al) - Integrated analysis reveals FOXA1 and Ku70/Ku80 as targets of ivermectin in prostate cancer
Pancreatic Cancer:
2022 Lee et al- Ivermectin and gemcitabine combination treatment induces apoptosis of pancreatic cancer cells via mitochondrial dysfunction.
2025 Morinaga et al - Ivermectin Combined With Recombinant Methioninase (rMETase) Synergistically Eradicates MiaPaCa-2 Pancreatic Cancer Cells.
Melanoma - (2022 Zhang et al) - Drug repurposing of ivermectin abrogates neutrophil extracellular traps and prevents melanoma metastasis
Liver Cancer - (2022 Lu et al) - Ivermectin synergizes sorafenib in hepatocellular carcinoma via targeting multiple oncogenic pathways
Stomach Cancer - (2021 Rabben et al) - Computational drug repositioning and experimental validation of ivermectin in treatment of gastric cancer
Esophagus Cancer - (2020, Chen et al) - Ivermectin suppresses tumour growth and metastasis through degradation of PAK1 in oesophageal squamous cell carcinoma
Kidney Cancer - (2017 Zhu et al) - Antibiotic ivermectin preferentially targets renal cancer through inducing mitochondrial dysfunction and oxidative damage
Animal studies have shown tumor volume reductions ranging from 50% to 85% depending on cancer type and dosing.
Related: Ivermectin Cancer Success Stories: Case Series Compilation (More than 200 cases)
Ivermectin Access and Controversies
Expert Opinion: Dr. William Makis
Dr. Makis emphasizes that Ivermectin has shown anti-cancer activity against over 20 types of cancer. However, due to its low cost and off-patent status, clinical trials remain unlikely. Studies in mice have demonstrated effectiveness in breast, colon, glioblastoma, glioma, and leukemia, but more research is needed for lymphoma, testicular cancer, and sarcomas.
IVERMECTIN acts on Cancer mainly by inhibiting signaling pathways involved in cancer proliferation (Akt, Wnt, mTOR) and also by inhibiting CANCER STEM CELLS.
Access to Ivermectin
In many countries, Ivermectin is available over the counter. However, regulatory bodies often impose restrictions, as seen in cases where physicians were penalized for prescribing it. Advocates argue that such restrictions deny patients access to potentially life-saving treatments.
Fenbendazole: Key Resources
Fenbendazole, another antiparasitic, exhibits at least 12 anti-cancer mechanisms. Studies indicate its effectiveness against triple-negative breast cancer, colon cancer, glioma, and leukemia. The ketogenic diet may enhance its therapeutic effects.
Oct.3, 2023 - FENBENDAZOLE and CANCER - at least 12 Anti-Cancer mechanisms of action. Not approved by FDA. Cheap. Safe. Kills aggressive cancers. Why no Clinical Trials? Nine research papers reviewed.
2023 - 2024 Fenbendazole Studies
2024 Apr, Rodrigues et al - Repurposing mebendazole against triple-negative breast cancer CNS metastasis
2024 Feb, Eid et al - Investigating the Promising Anticancer Activity of Cetuximab and Fenbendazole Combination as Dual CBS and VEGFR-2 Inhibitors and Endowed with Apoptotic Potential
2024 Feb, Park et al) - The microtubule cytoskeleton: A validated target for the development of 2-Aryl-1H-benzo[d]imidazole derivatives as potential anticancer agents
2024 Jan, Matsuo et al - Parbendazole as a promising drug for inducing differentiation of acute myeloid leukemia cells with various subtypes
2023, Dec, Iragavarapu-Charyulu et al - A novel treatment to enhance survival for end stage triple negative breast cancer using repurposed veterinary anthelmintics combined with gut‑supporting/immune enhancing molecules
2023 Nov, Aliabadi et al - In vitro and in vivo anticancer activity of mebendazole in colon cancer: a promising drug repositioning
2023 Nov, Jung et al - Fenbendazole Exhibits Differential Anticancer Effects In Vitro and In Vivo in Models of Mouse Lymphoma
2023 Sep, Garg et al - Network pharmacology and molecular docking study-based approach to explore mechanism of benzimidazole-based anthelmintics for the treatment of lung cancer
2023 Jun, Mukherjee et al - Ketogenic diet as a metabolic vehicle for enhancing the therapeutic efficacy of mebendazole and devimistat in preclinical pediatric glioma
2023 Feb, Lee et al - Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine
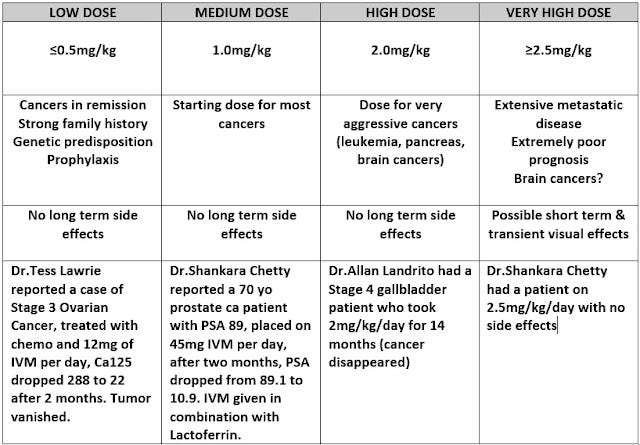
Experimental Cancer Protocols
For aggressive cancers, high-dose Fenbendazole (444-888mg/day) or Mebendazole (up to 4g/day) may be considered. Clinical trials support Mebendazole’s safety at high doses.
You can look at fenbendazole.org for suggested dosing and dose calculator.
Dr.Tom Rogers, Founder of “Performance Medicine” has similar protocols.
For anyone COVID-19 mRNA Vaccinated diagnosed with cancer (Turbo Cancer), I’d probably be looking at starting at 444 mg a day.
For particularly aggressive Turbo Cancers or bad cases, I’d even consider pushing towards 888 mg/day (444 mg twice a day) or 1000 mg/day.
Highest dosing I’ve seen is 30-50mg/kg/day for 5 days, based on the “Merck Manual”, however very few claim to have taken this dose.
Fenbendazole can elevate liver function tests, so it would be a good idea to have a family doctor monitor those.
Related: Fenbendazole cancer success stories (As of August 2025: More than 200 case reports)
Mebendazole: Key Resources
Here are the references for this dosing schedule:
For Maximum dose of 4g/day being safe, that’s from a Phase 2 Clinical Trial for Gastrointestinal Cancer: (2021 Mansoori et al)
(2021 Chai et al) - summarizes the various studies that have looked at Mebendazole in Cancer and the doses used.
500mg-1500mg/day (Phase 1 Clinical Trial, pediatric brain tumors)
200mg/day (2011 Dobrosotskaya et al) (adrenocortical ca)
200mg/day (2014 Nygren et al) (colon ca lung and LN mets)
100mg/day (Clinical Trial, UK)
So far, several studies in the literature have used 200mg/day with some success, however given that it is safe to go up to 4g/day, when we’re dealing with aggressive mRNA Induced Turbo Cancers, 200mg/day is probably not sufficient.
Why Mebendazole over Albendazole (2021 Chai et al):
“However, because of the toxicity of albendazole, for example, neutropenia due to myelosuppression, if high doses are used for a prolonged time, mebendazole is currently more popularly used than albendazole in anti-cancer clinical trials.”
New & Improved Joe Tippens Protocol
Below is a modified version based on the Joe Tippens protocol, a synergistic combination of fenbendazole, ivermectin and nutraceuticals, updated based on the ivermectin and mebendazole based protocol published in the Journal of Orthomolecular Medicine (2024):
Ivermectin (24 mg, 7 days a week) or in the case of severe aggressive cancers up to 1mg/kg/day.
Mebendazole (Dose of 200 - 400 mg/day) or Fenbendazole, commonly taken at 300 mg for six days a week, with doses increasing to up to 1 gram in cases of aggressive cancers.
Vitamin D (62.5 mcg [2500 IU] seven days a week).
Bio-Available Curcumin (600 mg per day, 7 days a week).
Enhanced absorption Berberine (500 mg per day) to starve your cancer of sugar.
Diet and Lifestyle: Eliminate sugar consumption as supported by the BMJ 2023 umbrella review, which recommends reducing free and added sugars to below 25 g/day and limiting sugar-sweetened beverages to less than one serving per week to reduce adverse health effects. Adopt a whole-food diet and avoid ultra-processed foods, as recommended by the BMJ 2024 guidelines. Additionally, prioritise adequate sleep and effective stress management to support overall health.
Notes:
Please note that this protocol now includes the vital Vitamin D addition, with the one day off for the fenbendazole administration. This protocol represents the most comprehensive and cutting edge repurposed drug and vitamin treatment approach to date.
If you are taking ivermectin and mebendazole, you might not need fenbendazole. Consult your doctor.
Vitamin E: Removed from the protocol (Joe Tippens, July 22, 2020) due to interactions (e.g., with blood thinners).
Ivermectin can significantly increase the blood-thinning effect of warfarin (Coumadin), and combining them increases the risk of dangerous or unusual bleeding. It may also interact with other anticoagulants, and patients should be closely monitored by a doctor.
Ivermectin works best when taken with high-fat foods.
Fenbendazole and Mebendazole Cost
Fenbendazole is cheap. If big pharma is going to make money (especially in cancer treatment), they need an expensive compound and Fenbendazole isn’t it.
Fenbendazole: Not FDA approved for cancer. Inexpensive
Mebendazole: FDA approved, costly. See: "Fenbendazole vs Mebendazole for Cancer".
Albendazole is FDA approved, expensive.
Dr. William Makis’s Take on Fenbendazole & Mebendazole
While research on Fenbendazole and cancer continues, scientists are increasingly focusing on other compounds in the Benzimidazole family, including Mebendazole, Albendazole, and Parbendazole.
If I were diagnosed with mRNA-induced turbo cancer as a male in my 40s, I would strongly consider a combination of Ivermectin (1mg/kg/day) and Fenbendazole (444mg/day). This decision would be based on numerous peer-reviewed studies, ongoing clinical trials, and existing research.
"Everyone’s situation is different, but it is crucial to arm yourself with medical knowledge that oncologists may not provide, either due to a lack of awareness or fear of professional risk."
Research Gaps and Future Directions
We recognize that double-blind, prospective, randomized controlled trials (RCTs) remain the gold standard in medical research. However, alternative study designs, such as N=1 trials, open-label studies, and real-world data analysis, provide practical insights—especially for rare or advanced cancers where large-scale RCTs may be impractical due to cost and time constraints. While these methods lack the rigorous controls of RCTs and are more susceptible to bias and confounding factors, they still offer valuable evidence that should not be overlooked.
For patients with Stage 4 or aggressive cancers, considering all available treatment options is essential, given the high-risk, high-reward nature of their condition. In such life-threatening scenarios, patients should have the "right to try" experimental or off-label treatments if standard options have been exhausted.
While clinical guidelines are informed by research, real-world patient care is not always dictated by clinical trials alone. Personalized treatment can be seen as a series of N=1 trials, where multiple interventions are carefully tested on an individual basis. By combining empirical evidence, clinical expertise, and real-time assessments—including cancer markers and PET scans—physicians can closely monitor treatment responses and adapt strategies quickly to maximize both effectiveness and safety.
Conclusion
Ivermectin, Fenbendazole, and Mebendazole show promise as experimental cancer treatments, supported by emerging research across multiple cancer types. However, clinical trial data remain limited. Patients should consult healthcare professionals before pursuing these protocols.
References:
Mel Gibson had 3 Friends cure their Stage Four Cancer with Fenbendazole and Ivermectin
Fenbendazole: Questions Answered, Things to Know, Useful Tips
Read More: This article is part of the Winning the War on Cancer series.
The Wellness Company's Ivermectin and Mebendazole
Ivermectin and mebendazole, both approved for human use, are now available in the U.S.
Researched and approved by Dr. Peter McCullough.
Prescribed by licensed medical professionals
Compounded and dispensed by a licensed US-based pharmacy
Approved for human use
Where to buy Ivermectin and Mebendazole Formula: Available on The Wellness Company's website. Here is the link: Ivermectin and Mebendazole.





I’m doing it — Stage 4 98% in remission and holding for 15 months now. New here 👋 Just published my first post Breast Cancer SmakDown. Would love your feedback! https://bcasmakdown.substack.com/p/bca-breast-cancer-smakdown-is-live?r=6dl5dr&utm_campaign=post&utm_medium=web
Thank you for sharing!